By Charlotte Webster-
British scientists have succeeded in creating a ‘synthetic’ embryo which developed a brain and a beating heart.
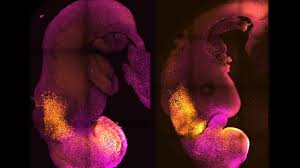
The research team in the UK and U.S has accomplished the incredible feat of creating “synthetic” mouse embryos that went on to develop a brain, a nerve cord and beating heart tissue in the lab without the need for a fertilised egg or uterus for it to grow in.
The incredible breakthrough is expected to revolutionise our insight into how a few cells go on to organise themselves into life.
Scientists are exploring the depths and limitations of its potential application to human embryos, which could aid understanding of human fertility, developmental disorders and provide a new avenue to develop tissues, or organs, for transplantation grown in the lab..
“The big question we’re addressing in the lab is how do we start our lives?” says Professor Magdalena Zernicka-Goetz from Caltech in Pasadena, California, and the University of Cambridge in the UK.
In creating the synthetic embryos, or “embryoids”, the scientists took three types of stem cell from a mouse embryo which would normally go on to form all the tissues required in a growing embryo.
They then transferred the cells into an artificial growth medium – essentially a rotating flask of nutrients. The creation comes from a team of researchers from Cambridge University, who used a combination of stem cells from mice following a decade of studying the early stages of pregnancy.
Scientists say that in order to be successful, pregnancies need a ‘dialogue’ between an embryo and mother. In the first week after fertilisation, three types of stem cells develop; one of which becomes the tissues of the body while the others support the embryo’s development.
Each group has to send mechanical and chemical signals to each other to tell the embryo how to develop properly, but if this period goes wrong, the pregnancy will fail.#

mouse embryos Image:cam.ac.uk
Scientists induced the expression of a particular set of genes and established a unique environment to encourage them to communicate. After self-assembling into an embryo, the researchers found they signalled chemically, mechanistically and through touch.
There were no fertilised eggs and no sperm, but the model copied the stages of mouse embryo development that take place up to eight-and-a-half days after fertilisation.
Lead author Professor Magdalena Zernicka-Goetz explained: “Our mouse embryo model not only develops a brain, but also a beating heart, all the components that go on to make up the body.”
“It’s just unbelievable that we’ve got this far,” she continued. “This has been the dream of our community for years, and major focus of our work for a decade and finally we’ve done it.”The embryo started to grow muscles, a gut and nervous system, which in turn offer insight into how tissues form and the causes of genetic diseases.
Prof Zernicka-Goetz said: “This period of human life is so mysterious, so to be able to see how it happens in a dish – to have access to these individual stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening – is quite special.
“We looked at the dialogue that has to happen between the different types of stem cell at that time – we’ve shown how it occurs and how it can go wrong.”
The professor also noted the model ‘allows us to manipulate genes to understand their developmental roles in a model experimental system’.
As a result, the world-first creation could help solve the donor shortage crisis and prevent miscarriages. Following the success of the mouse embryo model, the researchers are now developing similar human models with the potential to generate specific organ types.
The professor explained: “There are so many people around the world who wait for years for organ transplants. What makes our work so exciting is that the knowledge coming out of it could be used to grow correct synthetic human organs to save lives that are currently lost.
“It should also be possible to affect and heal adult organs by using the knowledge we have on how they are made. This is an incredible step forward and took ten years of hard work of many of my team members – I never thought we’d get to this place.
“You never think your dreams will come true, but they have.”
Early Human Embryo
The team are proposing synthetic embryoids that replicate just one element of an early human embryo, the heart for example, or the tissue that forms the placenta during implantation. Failure at implantation is a major reason for failure of IVF pregnancies.
Synthetic human embryos could also be used to generate new tissues or organs for generating new tissues or organs.
UK law also prevents human embryos from being grown in the laboratory beyond 14 days. This is earlier than most of the important developmental processes seen in these mouse embryos occur.
This latest demonstration means that discussion of these legal and ethical questions should start sooner rather than later, say experts.
“The result does herald that, in the future, similar experiments will be done with human cells and that, at some point, will yield similar results,” says Prof Alfonso Martinez Arias of Universitat Pompeu Fabra in Barcelona who was not connected with the research.
“This should encourage considerations of the ethics and societal impact of these experiments before they happen,” he added.
Earlier this month, researchers led by Jacob Hanna at the Weizmann Institute of Science in Israel announced they had made synthetic mouse embryos similar to real embryos 8.5 days after fertilisation by growing embryonic stem cells alongside two other kinds of helper cells. These were made by genetically altering embryonic stem cells to turn them into placenta-forming cells as well as a third kind of tissue called the endoderm, which normally directs development.
In the later stages, the structures were cultured in a special rotating incubator with raised oxygen pressure.
Now, Zernicka-Goetz and her team are proud of themselves after a similar feat, also using Hanna’s incubator, although they sourced the two kinds of helper cells by taking them from other embryos. Their synthetic embryos also resembled real 8.5-day-old embryos. After this stage, the synthetic embryos start to die, but the teams are trying new approaches to help them survive longer.
Zernicka-Goetz says the part that becomes the brain is more advanced than in any previous approaches as it includes the developing forebrain. “This is the very first model system that can develop all parts of the future brain,” she says.